
Cherry powdery mildew (caused by the fungus Podosphaera clandestina) is one of the most serious diseases in the Pacific Northwest. Despite the extensive cultural and fungicidal management of the pathogen, occasionally the conidia (asexual spores) are present on the surface of harvested fruit. This phenomenon is cause for concern in worldwide markets where the pathogen is absent.
According to the Northwest Horticultural Council, about one-third of the cherries produced in the Pacific Northwest are exported to other countries, including Australia, Japan, South Korea and New Zealand. Cherries produced in the region are fumigated as a quarantine routine before they enter the aforementioned markets.
The causal organism P. clandestina is a quarantine pest and therefore cherries must be free of any viable powdery mildew inoculum on the fruit surface. To that end, we investigated the efficacy of postharvest treatments for removing or killing conidia on fruit surfaces.
Typically, cherries undergo hydrocooling in chilled chlorinated water immediately after delivery to the packaging facility. Later, cherries are fumigated with methyl bromide at the correct pulp temperature of 8.3 to 10 degrees Celsius, (47 to 50 degrees Fahrenheit) as per the protocols defined by the country of export. For example, in the case of Australia, fumigation is carried out for two hours with 72 grams per cubic meter of methyl bromide at pulp temperature of 8.3 degrees Celsius or greater, and no more than 30.1 percent fumigation chamber load. After the fumigation, the cherries are routed onto the packaging line where fruit is washed in the mildly chlorinated, chilled, running water.
The cherries are then held and/or shipped under refrigeration (1 degree Celsius or 34 degrees Fahrenheit) to the destination countries. To determine the efficiency of each postharvest treatment, we harvested cherries with visible powdery mildew colonies (signs). The cherries were then subjected to industry-standard protocols for hydrocooling, fumigation and storage. The cherries were loaded onto the packaging line and stored for two additional weeks at 1.1 degrees Celsius to simulate international shipping. Cherries were sampled at each stage and conidia were retrieved using published laboratory protocols.
Using highly sensitive PCR techniques, we found that hydrocooling alone reduced the number of total conidia by 70 percent, while 98 percent of live conidia were removed. After fumigation, only 0.06 percent of the total conidia were viable. The experiment was repeated in 2019, with results similar to 2018.
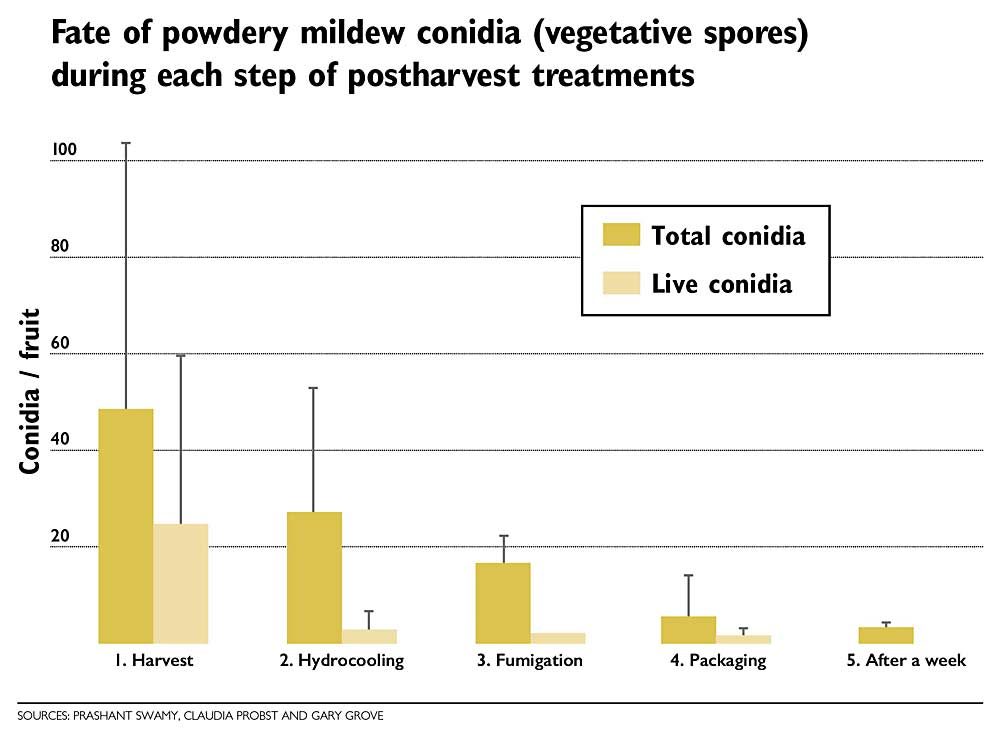
We also found that residual conidia isolated after the postharvest treatments were unable to establish powdery mildew colonies on healthy Bing cherry leaves, suggesting that the cherries do not carry any viable inoculum capable of infecting cherries in the country of their destination [1].
The chances of importation of viable P. clandestina propagules on cherry fruit are negligible because the fruit undergoes various postharvest treatments that are quite robust in removing and/or killing all conidia on the fruit surface. The chances of effective distribution of the pathogen are also negligible because viable inoculum is not found at the end of postharvest treatments.
Furthermore, the distribution of the pathogen would require the coincidence of susceptible sweet or sour cherry foliage and/or fruit, disease-conducive environmental conditions, and viable propagules of the pathogen in orchards in the country of destination. All of these conditions must be met for the establishment of the disease, which is extremely unlikely in the destination country of Australia since the host trees would be dormant in many areas and physical barriers (such as desert and mountains) render dispersal of the pathogen unlikely.
It should be noted that the pathogen viability decreases rapidly when the fruit is harvested. Therefore, the harvested fruit cannot provide an appropriate niche for the growth and reproduction of the pathogen that can subsequently become a potent inoculum source.
Overall, we demonstrated that the postharvest treatment practices in the Pacific Northwest remove all of the pathogen propagules from the fruit surface; even if a small trace of viable conidia are retained, the disease cannot be established in a destination country due to the lack of coincidence of the host, pathogen and environmental factors required to result in infection.
The research was initiated in 2016 with necessary proof-of-concept studies and all protocols were standardized before the actual experiment. The research was accomplished during the 2018 growing season and repeated in 2019.
The postharvest treatments, hydrocooling, fumigation, packaging and fruit storage were carried out at Zirkle Fruit Co. in Prosser, Washington. We thank Dave Copeland of Zirkle for his help in carrying out the experiments and Neusa Guerra for her technical assistance.
The funding for this research was provided by Washington State University, Washington Tree Fruit Research Commission and the Oregon Sweet Cherry Commission. •
—by Prashant Swamy, Claudia Probst and Gary Grove
Prashant Swamy is a research associate at Washington State University’s Irrigated Agriculture Research and Extension Center in Prosser, working with plant pathologist Gary Grove. Claudia Probst is a former research assistant professor at IAREC.
Reference
1. Swamy, P., C. Probst, and G.G. Grove, Incidence of Podosphaera clandestina on sweet cherries (Prunus avium) and the influence of postharvest handling practices on the survival of conidia on harvested fruit. Postharvest Biology and Technology, 2019. 156: p. 110929.






Leave A Comment