If you have sat through a food safety course, you’ve heard that we need to clean and sanitize food contact surfaces in order to decrease the chance of spreading foodborne pathogens. But, a lot of questions remain around how effective sanitizers are on the food contact surfaces used in harvest. Most sanitizers are only labeled for nonporous surfaces like hard plastic, leaving us with questions about efficacy for other surfaces such as those with a weave, like the Cordura nylon used in picking bags, or wood bins.
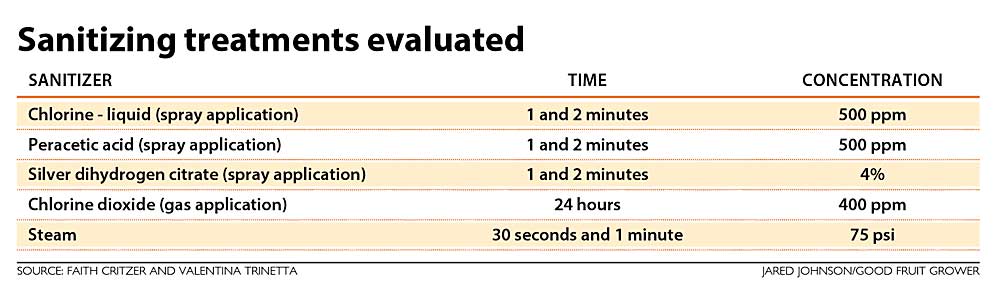
To help answer these questions, we first evaluated the activity of sanitizers in a lab setting with Listeria monocytogenes, Salmonella and Shiga toxin-producing E. coli that were inoculated onto surfaces. We also allowed these organisms to form biofilms as a worst-case scenario. We evaluated sanitizer treatments with Cordura (the heavy-duty fabric commonly used in the body of picking bags), high-density polyethylene plastic (HDPE) and plywood surfaces as detailed in the table.
Chlorine (sodium hypochlorite) and peracetic acid have been some of the most widely used sanitizers across the industry. They are both available in liquid forms that can be diluted on site and applied either as a spray or by immersing in a sanitizer solution. We utilized higher concentrations for these studies — at 500 parts per million — due to the fact we were utilizing a highly resistant form of microorganism in the biofilm and were also treating a porous surface (wood). The products used were labeled by the U.S. Environmental Protection Agency for sanitizing food contact surfaces at these concentrations. Not all products with the same active compounds have the same label, so make sure to check the maximum concentrations for use at your own farm or facility.
We also wanted to evaluate a few lesser-known options to see how they would perform:
—Silver dihydrogen citrate is a ready-to-use solution of 4 percent stabilized silver ions. The fact that it is ready to use may be a good fit for operations that do not want to mix a concentrated sanitizer.
—Chlorine dioxide is an antimicrobial gas that is typically dissolved in water and is used in some packing houses for washing produce, but it can be used in a gaseous state as well. All surfaces must be placed in a closed container or room to prevent employee exposure to the gas generated. Treatment occurs over 18–24 hours: When a precursor and activator are mixed, chlorine dioxide begins to form, builds and then dissipates within the time frame. The treatment is scalable to large and small situations since amounts of precursor and activator will be changed depending on the volume of space treated. Fans can be utilized for appropriate mixing in larger areas.
—Steam generators can inactivate microorganisms when temperatures are sufficient to cause their death. To achieve rapid inactivation, this often requires boosting the temperature of the surface you are sanitizing, heating it to over 120 degrees Fahrenheit, which requires a lot of energy.
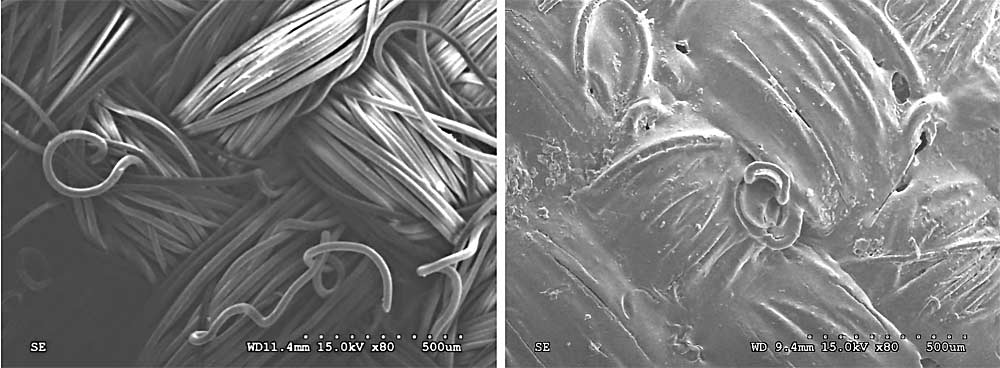
We were not surprised that wood proved to be one of the most challenging surfaces among the three evaluated, but we did not predict that Cordura nylon would be equally hard to sanitize in many cases. The images (at top) obtained by scanning electron microscopy show, on the left, Cordura alone in very high resolution — note the amount of surface area created by the fibers and covered area from the weave. On the right is the same surface but seen after a biofilm has formed on it over 96 hours — note the “film” distorting the weave, this is the biofilm. Hard plastic was consistently the easiest surface to sanitize, but even it can easily transfer microorganisms if not regularly cleaned and sanitized.
In the lab-based trials, additional exposure time did not significantly improve efficacy within the time frame evaluated. When bacterial foodborne pathogens were inoculated and immobilized on a food contact surface and then treated, they were successfully reduced by at least 99 percent in multiple treatments for all contact surfaces. Far less inactivation was observed when cells were allowed to grow into a biofilm before sanitation.
This is why it’s a key concept to have cleaning and sanitizing events at sufficient frequencies so that biofilms do not form: It’s much easier to inactivate an immobilized microorganism. We found chlorine dioxide to be one of the more effective treatments against biofilms on food contact surfaces. Steam was the least effective treatment.
Given that the industry is already using peracetic acid and chlorine-based sanitizers, we elected to evaluate the efficacy of chlorine dioxide and silver dihydrogen citrate with packing house cooperators in Washington, Iowa and Kansas, given that the products are newer to the industry and showed some efficacy in a controlled lab setting. Since we do not expect cooperators to have high populations of our target organisms (nonpathogenic E. coli and Listeria, especially as biofilms) we elected to bring small food contact surface samples that were inoculated with each form of the bacteria and affixed them to bins and picking bags at industry cooperators’ facilities.
Picking bags were stacked and treated in sealed totes, and bins were tarped in plastic to capture the chlorine dioxide generated. A sensor monitored the resulting concentrations of the sanitizing gas. Silver dihydrogen citrate was sprayed on bins and picking bags and allowed to drain. Chlorine dioxide treatments resulted in 90 to over 99.99 percent inactivation of target organisms, with the greatest reductions on plastic and the least on wood.
In the Midwest, where relative humidity in the treatment room was higher, greater inactivation was observed, highlighting the potential need to increase relative humidity if utilizing gaseous chlorine dioxide treatments in arid regions. Silver dihydrogen citrate performed better inactivating Listeria but had limited efficacy on E. coli. It also left a white residue — not on the bins or picking bags but where the solution drained, which could be easily tracked in a packing house setting.
When thinking about how you can apply this to your operation, keep surfaces cleaned and sanitized sufficiently so biofilms do not form. Don’t forget about picking bags. Keep to a defined cleaning and sanitizing schedule that will allow bags to fully dry before the next use. Wood can still be sanitized, but the sanitizers are far more effective on plastic. Similar to picking bags, bins need to be cleaned and sanitized before use. Train your crews to evaluate bins before they start to pick and to never pick into a dirty bin. If you are interested in learning more, please get in touch. We will be publishing these findings in the coming year and would be happy to share more details.
—by Faith Critzer and Valentina Trinetta
Faith Critzer is an associate professor at the University of Georgia. Valentina Trinetta is an associate professor at Kansas State University. They can be reached at fcritzer@uga.edu and vtrinetta@ksu.edu, respectively. This research was supported by the Center for Produce Safety and Washington State Department of Agriculture.






Leave A Comment