New Jersey’s tiny wine industry is booming — as are local wineries across much of the Eastern U.S. — but grape growers in those regions face increased disease pressure from fungi such as downy mildew that thrive in the humid conditions.
That’s why a plant pathologist at Rutgers University is experimenting with a cutting-edge tool to basically turn off the genetic welcome mat in the grape’s DNA that allows the downy mildew fungus to take hold.
If successful, the research will create Chardonnay grapes that resist the pathogen and that could hopefully be grown without dependence on intensive fungicide application.
That’s very appealing to some of the region’s growers because winemakers are often concerned about how pesticide residues may impact fermentation, said Dan Ward, director of the New Jersey Center for Wine Research and Education.
Currently about 200 wine grape growers, working over 2,000 acres in New Jersey, rely on careful canopy management to allow air circulation and light penetration to combat wet conditions that encourage fungal disease, Ward said.
Even with those techniques, Eastern U.S. grape growers are completely dependent on fungicides to prevent downy mildew and other common diseases from destroying crops and eventually killing the vineyards. That’s why Ward is excited about the potential of engineering resistant grapes.
“I brought it up at (industry meetings) and the people who responded were excited by the prospect of a genetically modified grapevine being available to them to reduce the need for fungicide,” Ward said. “There is interest among some of our larger grape growers, who were immediately warm to the idea of modifications to an existing variety — preserving the qualities that make them valuable while eliminating the qualities that make them susceptible.”
No open window for mildew
Rong Di, a professor of plant biology at Rutgers, is growing tiny mutant plants in her lab that are resistant to the fungal pathogen. But she’s not actually growing grapes yet.
She started to test her theory that turning off the genes that serve as “host factors” for mildew would create disease resistance in an extremely well-studied little mustard known as Arabidopsis, which serves as a model in lots of genetic research.
These host factor genes basically give each specific fungus the ability to interact with and infect the host plant, Di said. To turn them off, she’s using a gene editing tool called CRISPR.
Praised as a biotechnology breakthrough, the powerful tool has been adopted by scientists around the country for its precise and efficient ability to edit the DNA of any organism.
“I’m always on the lookout for new technology, so three years ago when the CRISPR gene editing came out, I decided to try it,” Di said. Previously, she had used a different genetic engineering technique that could turn down — but not turn off — the expression of host factor genes in soybeans to make them more resistant to nematodes.
“We know if we knock down the interaction of the host factors, we know we can improve resistance, so if it’s completely knocked out, we can improve resistance even more,” Di said. “And now with gene editing technology, it’s much easier to knock out a gene.”
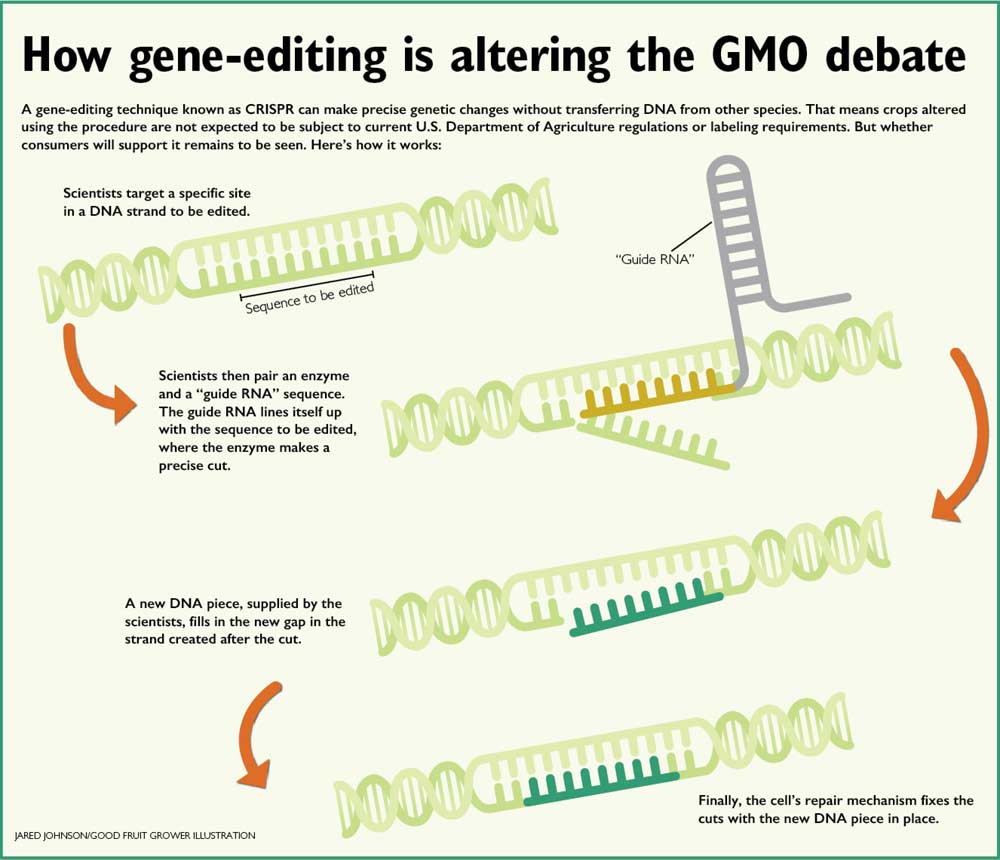
Gene-editing with CRISPR works by pairing an enzyme and a guiding piece of genetic code known as RNA to a specific site in the DNA, where the enzyme, known as an endonuclease, makes a precise cut.
Then, the cell’s own DNA repair mechanism puts a new DNA piece, supplied by the scientist, into the newly created gap. It could be an entirely new gene, or, as is the case in Di’s work, just a tiny change that renders the existing gene unable to be translated into the proteins that work as that welcome mat for invading fungi.
“It’s very targeted; we can direct the DNA endonucleases where we want so we don’t have to hurt the plant in other ways. We only knock out the gene I choose,” Di said.
The technique worked well in the Arabidopsis test, so Di has begun to use it to transform the comparable host genes in Chardonnay grape cells in her lab.
If it works as she expects it to, it won’t be hard to replicate the procedure to make other grape varieties resistant to downy mildew as well because the host factor genes are similar in each variety, Di said.
Since the sophisticated CRISPR technique requires no transfer genes from other species, like some traditional means of genetic engineering that have spurred controversy, it’s unclear at this time if crops altered this way will be considered genetically modified organisms by regulators.
And it remains to be seen if the wine industry in New Jersey and other regions where downy mildew is a significant problem will embrace Di’s genetically engineered grape. But Ward said that ending growers’ dependence on fungicides would have multiple benefits.
“It’s really the pursuit of reduced pesticide residue on the fruit from the consumers’ point of view and reduced need for applications from the producers’ point of view and reduced environment impacts of pesticides, which is a benefit from everyone’s point of view,” Ward said. “Wine grape growers as a group tend to be well educated and sophisticated folks … they are out there trying to optimize their use of any technology we develop.” •
– by Kate Prengaman






Leave A Comment